orange book pharmacy uk
The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book. Full name required Date of Birth required Post Code required.
The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange Guide is now available through MedicinesComplete.

. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been. The updates incorporate changes. Approved Drug Products with Therapeutic Equivalence Evaluations.
Rules and Guidance for Pharmaceutical Distributors 2022 The Green Guide 6378 7200. Originally this book was published in October 1980 with orange cover and thus the name orange book. Rules and Guidance for Pharmaceutical Manufacturers and.
The electronic availability of the Orange Book brings this valuable tool to the web for. As of May 2020. Book Library is an easy-to-use yet powerful book collection manager.
1 Generic substitution laws are. The orange book is published annually and the 2015 edition is 35th. The full publication title is Approved.
The Orange Book has long been a reliable resource for information about FDA-approved drugs. Good manufacturing practice GMP is the minimum standard that a medicines manufacturer must meet in their production processes. The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution.
The FDAs Orange Book or Approved Drug Products with Therapeutic Equivalence Evaluations lists products with their corresponding therapeutic equivalents. Data Descriptions updated February 24 2017 Orange Book Search You can search by active ingredient proprietary name applicant or application number. The Orange Guide is essential reading for anyone subject to MHRA inspection providing you with all the answers you need to stay informed.
It lets you catalog any printed materials such as books magazines comic books and newspapers with a. Rules and Guidance for Pharmaceutical Manufacturers and Distributors 2022 The MHRA Orange Guide MHRA Medicines and Healthcare products Regulatory Agency The MHRA Orange. Rules and Guidance for.
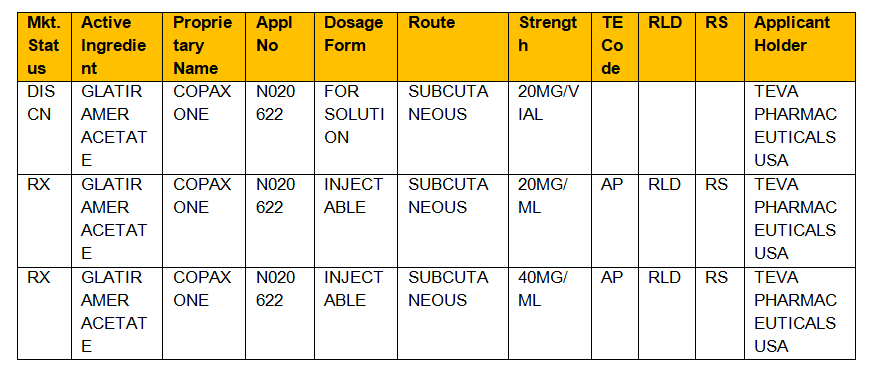
Orange Book And Its Applications Legal Advantage

Pharmaceutical Press Rules And Guidance For Pharmaceutical Manufacturers And Distributors 2022 The Mhra Orange Guide

New Essential Orange And Green Guides 2017 Out Now Gov Uk

Rules And Guidance For Pharmaceutical Manufacturers And Distributors 2017 The Orange Guide

Rules And Guidance For Pharmaceutical Manufacturers And Distributors 2022 The Orange Guide Pharmacy Books
Erle Stanley Gardner The D A Breaks A Seal London Cassell And Lot 94064 Heritage Auctions

9 28 Flu Vaccine Requirement For Students Usc Student Health

Top Screwups Doctors Make And How To Avoid Them By Teresa Graedon Book The Fast 9780307460912 Ebay
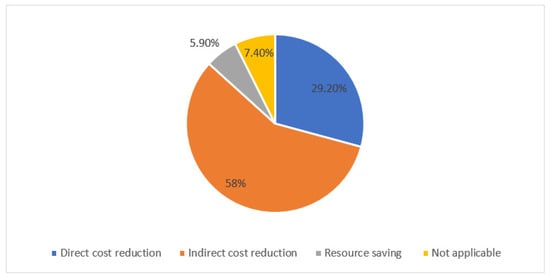
Pharmacy An Open Access Journal From Mdpi

Pharmacy Drawer Cabinet 19th Century For Sale At Pamono
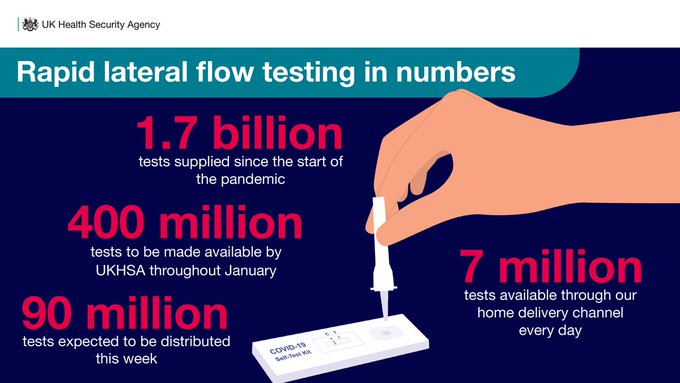
Free Rapid Tests In The U S Lag Behind Other Countries The Washington Post
Coronavirus Covid 19 National Kidney Federation

Covid 19 Local Information Possability People

Community Event The Poetry Pharmacy Wimbledon Community Association

Remington Science Practice Pharmacy Abebooks

The Maudsley Practice Guidelines For Physical Health Conditions In Psychiatry The Maudsley Prescribing Guidelines Series 9781119554202 Medicine Health Science Books Amazon Com



